Only applications from Japan-based Marketing Authorization Holders are acceptable and an application form and related documents must be written in Japanese.
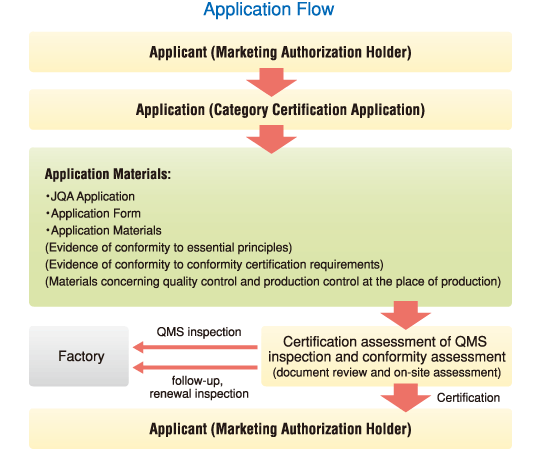
* Surveillance audits are conducted annually and a renewal audit will be conducted before the end of the validity term (5 years).
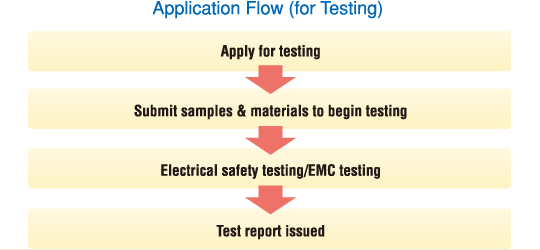
Custom test reports can be used in approval/certification applications and as testing data for internal use.